From tumor to therapy
When it comes to diseases, the best treatment is often as unique as the patient. This also applies to the respective tumor. Nevertheless, many cancer therapies today are still based on statistical averages. In the ‘photiomics’ Bosch Research project, Bernd Scheufele and his team developed a tumor bioreactor that enables personalized drug tests with real tumor tissue — outside the body, automated and standardized.
When a person is diagnosed with cancer, a race against time begins. Which therapy is effective? What helps on an individual, rather than on a statistical, basis? These questions defined the starting point of the ‘cells-on-chip technology’ Bosch Research project aimed at developing a technological solution for personalized cancer treatment. What began as a single person’s initiative in 2018 has matured into a functional tumor bioreactor that provides initial answers. “I used to work in clinical research,” says Dr Bernd Scheufele, interdisciplinary research physicist at Bosch Research. “Projects that directly help patients, their families and other people involved have always been particularly close to my heart,” he adds. While working at the University Hospital in Tübingen, he was already tackling the question of why cancer therapy is always based on a standard rather than focusing on the specific characteristics of the patients first and foremost. “Every person has unique genetic and tissue-specific characteristics, and every tumor is different.”
The interdisciplinary photiomics project
Today, cancer therapy is generally based on clinical guidelines, i.e., evidence-based and statistically derived average values. However, cancer is an individual disease. Tumors differ genetically as well as in terms of their immunological characteristics and their microstructure. This personalised approach paved the way for the photiomics joint project funded by the Federal Ministry for Research, Technology and Space (BMFTR) (grant number 13N15768). The ‘photiomics’ project name is an artificial word that combines the terms photonics and genomics. From 2022 onwards, Bernd Scheufele and his team collaborated with clinical, research and industrial partners (Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology at the Bosch Health Campus, Zellkraftwerk GmbH/Canopy Biosciences/Bruker Group, Raylytic Software GmbH) for around three years. The aim was to increase the success rate of drug therapy by selecting individualized medication before the start of therapy.
Building on the lab-on-chip technology already established at Bosch, Bernd Scheufele and his team are working on a new approach to personalized cancer therapy: a living tumor tissue slice that is automatically tested with drugs in a compact technical system. This system, the so-called tumor bioreactor, makes it possible to test the efficacy of substances based on an actual sample of the patient’s tumor tissue. This could help to avoid stressful and ineffective drug therapies and allow more effective treatments to be found earlier. “We use tissue that is removed during diagnosis or surgery anyway. Instead of just analyzing it histologically, we directly test how it reacts to various drugs,” says Bernd Scheufele.
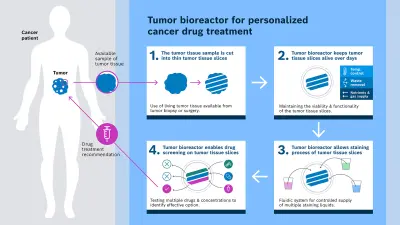
How the tumor bioreactor works
The question that got everything started may sound simple, but it is highly complex from a medical point of view: How can you predict which drug will actually work for a specific tumor — and which will not?
Complex problems are best solved in interdisciplinary teams whose members specialise in different fields. The photiomics project is no exception. Bernd Scheufele collaborated at Bosch Research with researchers from the fields of physics, biology, chemistry, fluidics, optics and systems engineering. The aim was to develop a functional tumor bioreactor capable of doing the following things: cultivate viable tumor slices over several days, perform automated fluorescence staining and drug treatment of living tumor slices.
The following three core processes take place in the tumor bioreactor: preservation of tissue vitality, color-coded reactions to identify different drug effects, as well as automated drug delivery. Unlike conventional methods, the tumor tissue is processed immediately after removal instead of being fixed or frozen. Gentle cutting with a vibrating knife produces living tissue slices with a thickness of around 150 to 300 micrometers that retain their natural cell structure. This requires the tumor tissue to be at least five millimeters in size. The Bosch Research team succeeded in keeping the tumor slices viable in the tumor bioreactor for several days. This is enough time to perform numerous drug tests. The tumor slices are cultivated by means of a precise fluid supply with nutrients, gas exchange, removal of waste products and temperature control.
The tissue slices provide cross-sections of the tumor tissue that contain its various layers and structures. In the tissue slices, fluorescent stainings mark cell properties so that even subtle reactions to drugs can be detected. At present, up to nine slices can be tested in parallel – with different substances or dosages. The effects are visible either directly in the tissue or by analyzing the fluid above the slices. Fluorescent markers can be used to visualize different cell types, biomarkers or reactions.
The researchers in the consortium developed the necessary infrastructure for these processes. The fluidic system of the tumor bioreactor, developed by Bosch Research, now enables the precise dosing of various drugs onto the tumor tissue slices at defined concentrations and volumes. In cooperation with the partners, the reactions are recorded using a microscope, and imaging and analysis begin.

The perspective of personalized therapy
Bernd Scheufele and his team are currently using the tumor bioreactor in extensive tests. In the long term, it could become a decisive diagnostic tool for personalized cancer therapy as a complement to conventional tumor boards. Furthermore, it could also be used for preclinical drug tests and in pharmaceutical research to develop new active ingredients. The advantages are obvious: fewer ineffective therapies, fewer side effects, higher probability of success.
For Bosch, this technology opens up a new field in medical diagnostics systems, particularly in the future market of functional drug testing using living tissue. The bioreactor has not yet been approved for clinical use. To make it viable for routine medical application, clinical studies are needed to prove that the predictive power of the system actually leads to better treatment decisions. Medical and clinical research must be carried out, which is time consuming but essential, says Bernd Scheufele.
The Bosch Research team also has its sights set on further technological developments: Scaling to more tumor slices (around 50 slices per tissue sample could be possible), improved image analysis using artificial intelligence and the testing of novel drug combinations. The platform could also be used for preclinical studies in the pharmaceutical industry, for example to identify drug resistances, side effects or combination effects, meaning the interaction of drugs that are administered at the same time. With the successful completion of the project in October 2025, one of Bernd Scheufele’s heartfelt wishes has already been fulfilled: “We have demonstrated that it is possible. Now we want to show what it can do.”
Profile

Bernd Scheufele
Bernd Scheufele leads the cells-on-chip technology initiative for personalized cancer treatment at Bosch Research. With a background in physics from the University of Tübingen and a PhD from the Technical University of Kaiserslautern, he has extensive experience in medical device development, microfluidics, and clinical sciences. Before joining Bosch, he worked in academia on blood flow analysis and later developed piezoelectric micropumps and lab-on-chip systems for medical and pharmaceutical use. At Bosch, he created a 3D ultrasonic sensor for automated driving and now heads the cells-on-chip and photiomics projects within the Strategic Portfolio Healthcare Solutions.