Combating the coronavirus pandemic: Bosch develops rapid test for COVID-19

Bosch has developed a new rapid test for its Vivalytic analysis device to detect the SARS-CoV-2 pathogen. The test provides a reliable result in 39 minutes and is currently the fastest polymerase chain reaction (PCR) test worldwide. Results for positive samples are even delivered in less than 30 minutes.
Bosch’s new rapid test is predestined for decentralized use in mobile test centers at freeway service stations or in airports. People who take the test can obtain a reliable result while at the testing site. Available now in Europe, the CE-approved test helps avoid time in quarantine, relieve laboratories, and make travel and work safer again. “One of the keys to fighting the coronavirus pandemic is to rapidly identify sources of infection. That’s why we focused on following up on our first coronavirus test with an even faster one,” says Dr. Volkmar Denner, chairman of the board of management of Robert Bosch GmbH. “This will enable us to put people’s minds at ease more quickly.” The development of the new Bosch PCR singleplex test is part of a research and development project funded by the German Federal Ministry of Education and Research (BMBF).
As the federal minister for education and research Anja Karliczek says, “I believe it’s important that people have clarity about their state of health as quickly as possible. In this respect, insights from science and research can bring people huge benefits. Over the next few months, we will be confronted with the particular challenge of having to test more people. The improved testing procedure developed by Bosch with the BMBF’s support has the potential to be a tremendous help with this complex job. The rapid improvement of our technological capabilities shows what innovative achievements German companies can deliver in times of crisis.”
The test has a sensitivity of 98 percent and a specificity of 100 percent. To develop it, the Bosch subsidiary Bosch Healthcare Solutions joined forces with the German biotechnology company R-Biopharm – a leading provider of highly sensitive manual PCR tests. PCR tests are considered the gold standard of test methods.

One of the keys to fighting the coronavirus pandemic is to rapidly identify sources of infection.
A world first: simultaneous testing of five samples

Bosch launched the first rapid test for its Vivalytic analysis device at the end of March 2020, after just six weeks’ development. As a multiplex test, it simultaneously checks patient samples for the SARS-CoV-2 virus and nine other respiratory diseases in under two and a half hours. The new, accelerated test is exclusively for SARS-CoV-2. “With our different coronavirus tests and variable analysis strategies, we open up a range of testing scenarios with a Vivalytic device — from screening all the way to supporting differential diagnosis for diseases with similar symptoms,” says Marc Meier, president of Bosch Healthcare Solutions GmbH. And development work is still in full swing at Bosch: As of early October 2020, it will be possible to simultaneously evaluate five samples in one test cartridge and at a comparable speed — a world first. Bosch is thus increasing available testing capacity, enabling fully automated processing of more than 160 samples a day using a Vivalytic device. In addition, optimized software will in the next few weeks further reduce the turnaround time of the SARS-CoV-2 test on positive samples.
More than 160 samples
a day can be processed fully automated.
Vivalytic analysis device: easy to use at the test site
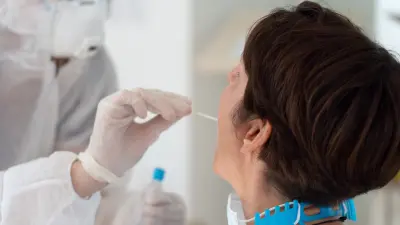
The advantages of Bosch’s rapid test lie not only in speedy analysis, but also in ease of use. A sample is taken from the patient’s nose and throat using a swab, and placed in the test cartridge. Then the test cartridge, which already contains all the reagents required for the test, is inserted into the Vivalytic device for automated analysis. The Vivalytic analysis device is designed to be user-friendly; medical staff require only brief training on how to operate it. The development of the Vivalytic system, which consists of an analysis device and test cartridges, grew out of a long-standing collaboration between Bosch’s corporate research and advance engineering and Bosch Healthcare Solutions.
As demand for the analysis device and the rapid tests remains high, the company is working closely with its suppliers to maximize capacity and further increase supply.
Updates from the cloud platform Vivasuite
Updating the Vivalytic testing devices is simple and straightforward – all that is required is an internet connection to access the Bosch cloud platform Vivasuite. Developed in-house at Bosch, the Vivasuite cloud platform allows users to digitally manage and update all their Bosch Healthcare Solutions devices. This is also an advantage when Vivalytic devices are in use in the field. The platform meets the strictest security standards and data privacy is guaranteed at all times: for example, there is no remote access to Vivalytic devices, and no possibility of accessing patient data.

