Treating cancer with personalized medication
Bosch Research Blog | Posted by Dr. Bernd Scheufele, 2023-05-31

Medical technology research for personalized drug treatment of cancer patients
Is it possible to improve the treatment of a cancer patient by selecting an appropriate individualized drug therapy?
At Bosch Research, my team and I are currently working on this challenging and very complex issue. We are investigating and developing innovative technological solutions for the personalization of cancer treatment by using a patient tumor sample to perform drug screening and determine the appropriate medication for the cancer patient.
For this objective, we collaborate with a strong consortium of academic, clinical, and industrial cooperation partners and are part of a research project funded by the German Federal Ministry of Education and Research. The established multidisciplinary cooperation is essential for the demanding research & development tasks and very beneficial in terms of the synergies.
My blog post introduces you to the research activity cells-on-chip technology for personalization of cancer therapy that I initiated and lead at the Bosch research campus in Renningen, Germany. The post will also give you some insights into the background and targeted novel approach to improving the efficacy of anti-cancer drug treatment.
The motivation and need for personalized cancer treatment
A cancer diagnosis changes a person’s life and those of their family and friends in a drastic way, as cancer requires complex treatment and is unfortunately still one of the most common causes of death. In Germany alone, about 500,000 people are newly diagnosed with cancer every year and appropriate treatment options must be selected for every cancer patient.
In recent decades, the treatment of cancer has evolved and improved significantly, so that cancer patients and medical professionals have a selection of powerful therapeutic approaches to choose from. There are currently several different options for cancer treatment, depending primarily on the type and stage of the cancer. For example, the treatment of solid tumors such as lung, breast, colon, liver, and prostate cancer can involve surgery, radiation therapy, and drug therapy. A commonly used form of drug therapy is chemotherapy, which uses agents that inhibit the rapid growth of cancer cells or destroy them. Other drug-based treatment approaches are targeted therapies, immunotherapies, and hormonal therapies.
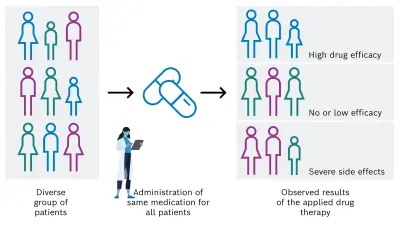
Despite great efforts in the development and clinical application of anti-cancer drugs, the individual response of a patient to a specific pharmacological cancer therapy can unfortunately vary strongly. In the positive case, the desired efficacy is achieved, but it can also be absent or too low. In addition, undesired serious side effects can occur because of the administered drug (see Figure 1A). Main reasons for the varying efficacy of anti-cancer medications include the individual variability of cancer, the heterogeneity of solid tumors, and the high complexity of the tumor microenvironment and pharmacological processes.
Currently, the accurate prediction of an individual patient’s response to established or novel anti-cancer drugs is an unsolved challenge in cancer treatment.
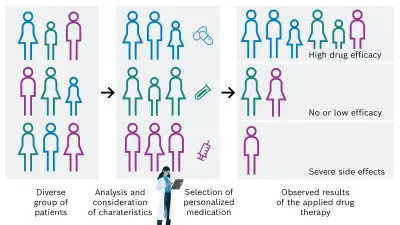
There is a high need for personalization of the drug-based cancer treatment of tumors in order to administer the adequate and successful individual medication for each cancer patient. This could be achieved by considering and analyzing specific patient characteristics, for example tissue, genetic, and lifestyle factors. On that basis, the most appropriate drug is then selected, resulting in a desired increase of drug efficacy and decrease of unintended side effects (see Figure 1B).
Improving cancer treatment by personalizing the medication
Solution approach to enable personalized drug treatment of tumors
As the response of a solid tumor to pharmacological treatment is very difficult to predict, a suitable personalized approach is needed to test potential medications outside the body, determine the most effective option, and derive an individual treatment recommendation for the cancer patient. For this objective, I launched the cells-on-chip technology research activity with external collaboration partners to develop a novel diagnostic solution and implement the concept shown in Figure 2.
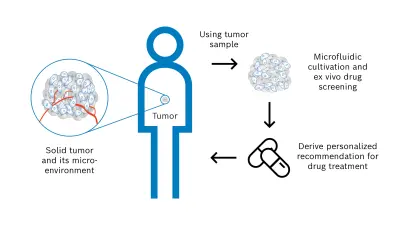
Steps of the personalized concept to assess individual drug sensitivity & resistance:
- (1) The diagnostic basis and starting point of the workflow is a tissue sample of the solid tumor, which is already available from a previously performed biopsy or surgical tumor resection. This tissue sample consists not only of malignant cells but also of the tumor microenvironment that significantly affects an anti-cancer drug treatment. The microenvironment comprises e.g. blood vessels, immune cells, signaling molecules, and the extracellular matrix.
- (2) A 3D tumor model is then derived from the tumor sample to conduct functional drug screening by a microfluidic solution. The model must resemble characteristic and relevant properties of the solid tumor. Therefore, the following two model systems are investigated – tumor tissue slices and tumor organoids.
Tumor tissue slices are produced by cutting thin layers of the viable tumor sample without preprocessing. This model has the advantage of preserving the high complexity of the solid tumor and tumor microenvironment accurately and quickly. Tumor organoids are produced by mechanical and enzymatic dissociation of the tissue sample followed by 3D cultivation of the resulting cells with an appropriate medium and hydrogel scaffold over several days. This model showed promising results in preclinical drug development. - (3) As next step, the tumor model is cultivated to ensure the required viability and functionality over time. In particular, nutrients and gases are supplied in a controlled manner. To perform the functional drug screening, different pharmacological agents with defined concentrations and volumes are applied to the tumor model. The pharmacological agents act on the tumor model for certain time periods, the agents are then removed, and the resulting effects of the drug treatment are analyzed in detail. This analysis is carried out for example by immunohistochemical staining and includes the evaluation of relevant biomarkers and alterations.
Finally, the drug screening results obtained outside the patient’s body enable the intended recommendation for the individualized cancer drug treatment. The diagnostic results are provided to the clinical oncologist who can make the decision on drug administration together with the patient from whom the tumor tissue sample was obtained.
For the targeted clinical use case and further industrial applications in the field of drug discovery and development, the standardization and validation of the tissue-derived tumor models in combination with the corresponding technological solution are essential. At Bosch Research, we are currently working on the development of an automated and miniaturized workflow implementation. As a planned next step, this innovative new diagnostic technology can be used to conduct required larger studies and prospective clinical trials.
Project funded by the German Federal Ministry of Education and Research
The research & development of an innovative technological solution for personalization of anti-cancer drug treatment by using a patient’s tumor sample for functional drug screening requires a broad range of different competencies and should be approached with interdisciplinary collaboration partners. Therefore, I am very glad that the German Federal Ministry of Education and Research (BMBF) is funding the proposed photiomics research project on this innovative topic (grant number FKZ 13N15768) and is giving us the possibility to work together with an excellent project consortium.
The main goal of the publicly funded photiomics project with industrial, academic, and clinical collaboration partners is a diagnostic platform for conduction and evaluation of individual drug screening on viable tumor slices from cancer patients as shown in the previous Figure 2. The project addresses the need to personalize drug treatment against cancer in which microorganisms are involved in tumor genesis. In particular, infection with hepatitis viruses can lead to the formation of liver cancer.
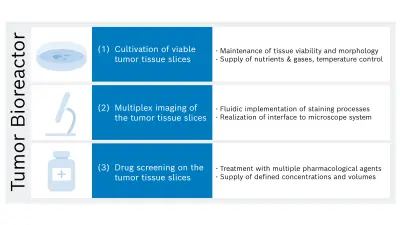
In the project, a novel analytical method will be developed to determine drug-induced effects using fluorescence imaging of cultivated liver tumor slices and image analysis enhanced with artificial intelligence. Bosch Research is specifically responsible for developing a bioreactor platform with three main functionalities (see Figure 3): cultivation, imaging, and drug treatment of several tumor slices with a thickness of approximately 150-300 µm.
More details about the publicly funded project photiomics, the established project consortium as well as the project volume and duration are summarized on this website.
What are your thoughts on this topic?
Please feel free to share them or to contact me directly.

Author: Bernd Scheufele
Bernd Scheufele is head and initiator of the cells-on-chip technology for personalized cancer treatment research activity at Bosch Research and has broad expertise in medical device development, medical and clinical sciences, and microfluidic systems. Bernd studied physics at the University of Tübingen, Germany with an interdisciplinary diploma thesis on blood flow in capillary blood vessels. Afterwards, he was scientist at University Hospital Tübingen and analyzed cerebral blood flow in newborns determined by ultrasound. He received a PhD in physics from the Technical University of Kaiserslautern, Germany for creating a novel method for piezoelectric antibody detection and a microfluidic platform for point-of-care blood analysis. As next step, he joined the medical device industry and developed piezoelectric micropumps and microfluidic systems for applications in medical technology, pharmaceutics, and life sciences. He later joined Bosch, created a 3D ultrasonic sensor for advanced automated driving, and transferred this sensor technology to the first startup of Bosch Business Innovations incubator for industrialization. He is currently part of Strategic Portfolio Healthcare Solutions at Bosch Research, and is leading the cells-on-chip activity and publicly funded photiomics project featured in this blog post.
Further Information
The following resources provide a more in-depth understanding of the investigated approach to personalizing anti-cancer drug treatment and other use cases of interest to Bosch.